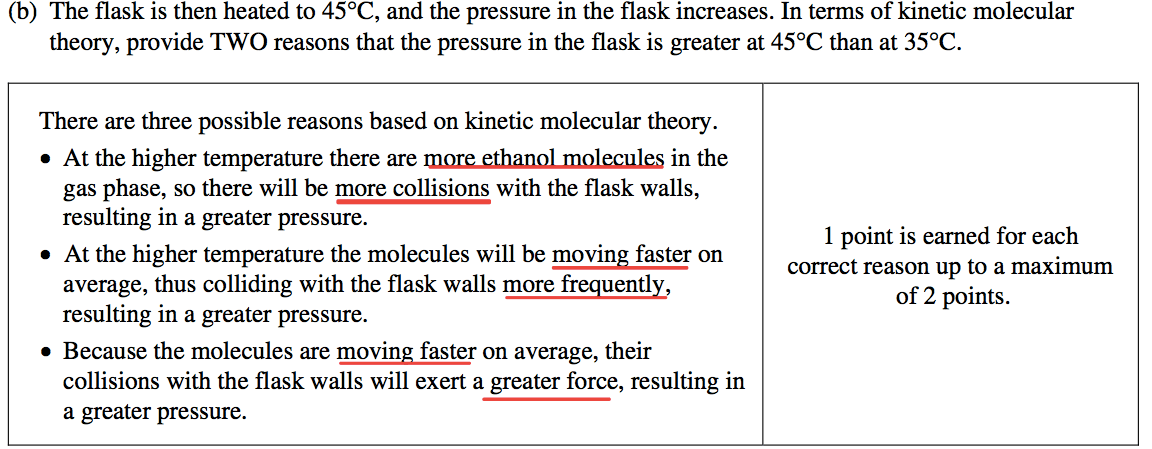
Question 1 (c)
![(ii) The contents of beaker 2 are poured into beaker 3 and the
resulting solution is stirred. Assume that volumes are additive.
Calculate the pH of the resulting solution. In the resulting solution,
\[NH3\] = \[NH'\] , Ka = 5.6 X 10-10 \[NH4+\] Thus \[H30+\] = 5.6 x
10-10; pH = -log(5.6 x 10-10) 1 point is earned for noting that the
solution is a buffer with \[NH3\] = \[NH'\]. 1 point is eamed for the
correct pH. = 9.25](media/image119.png)
Henderson–Hasselbalch equation
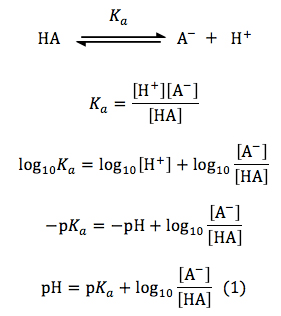
Question 1 (d)
![(ii) Calculate the final \[NH4+\] in the resulting solution at 250C.
moles = (volume)(molarity) moles H30+ in sol 1 . = = 0.00250 mol moles
NH3 in 2 = = 0.00250 mol moles NH4+ in sol 3 . = = 0.00250 mol When
the solutions are mixed, the H30+ and NH3 react to form NH + resulting
in a total of 0.00500 mol NH 4 +. The final volume is the sum
(25.0+25.0+25.0) = 75.0mL. The final concentration of NH + = (0.00500
mol/O.0750L) = 0.0667 M. 1 point is earned for the correct calculation
of moles of NH 4. 1 point is eamed for the correct calculation of the
final volume and concentration.](media/image121.png)
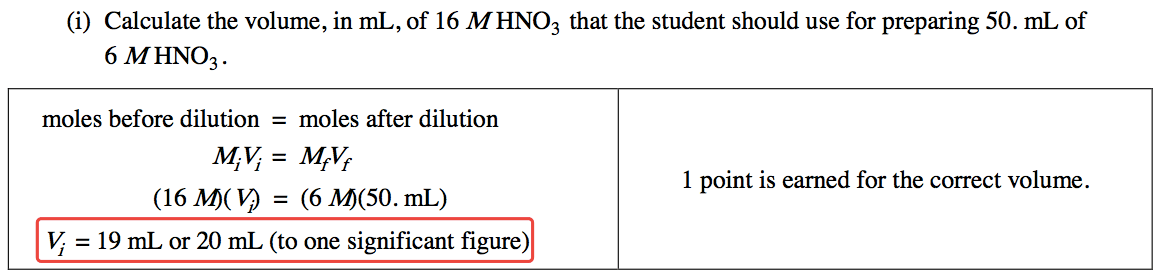
Question 2 (a)
Your answers should have the same decimal places as the equipment can read
Procedure for preparing solutions

Volumetric flask vs. Graduated cylinder
Question 3 (a)
Notice the coefficient of the reactants and products in calculating standard enthalpy change
Standard enthalpy of formation of pure elements in their standard states are assigned zero
![2 H2(g) + 02(g) 2 H20(1) (a) Calculate the standard enthalpy change,
AHO for the reaction represented by the equation above. 298 (The molar
enthalpy of formation, AH AH0298 = - \[2(0) + 1(0)\] ; , for H20(1) is
-285.8 kJ mol-I at 298 K.) = -571.6kJ mol-I 1 point is earned for the
correct answer.](media/image125.png)
Question 5 (b)
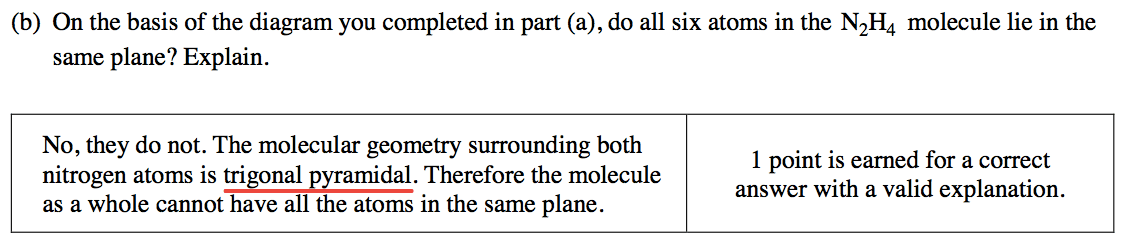
N2H4 molecular geometry
Trigonal pyramidal
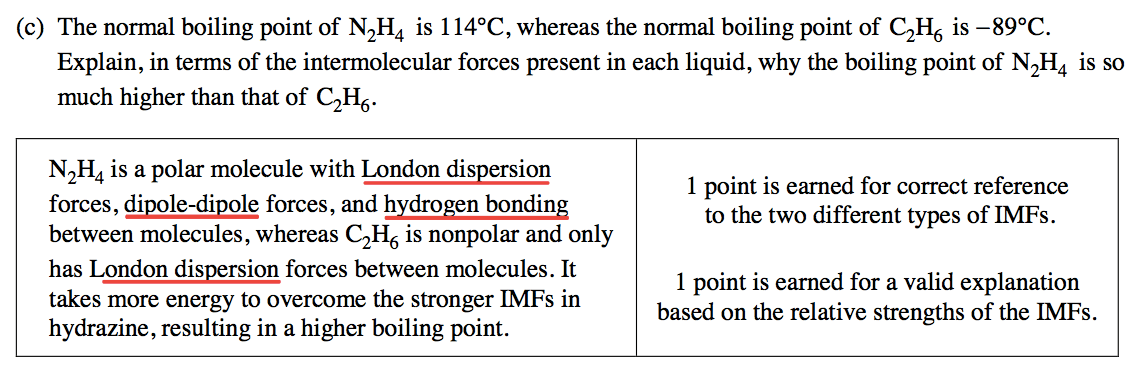
Question 5 (c)
Question 6 (b)